India is currently suffering through one of the worst COVID-19 crises. With a shortage of supplemental oxygen and hospital beds, the pandemic is starting to rear its ugly head. Nevertheless, a hopeful ray of sunshine has fallen upon this nation. The Defence Research and Development Organisation (DRDO) has released an anti-COVID drug that has been approved for oral emergency use.
However, the drug has raised some concerns among the public as they are missing some critical information.
On May 8th, India’s Ministry of Defence announced a press release for the drug. The Defence Minister and the Union Health Minister released the first batch of the anti-COVID (2-DG) drug on May 17th.
The therapeutic application of the drug has been developed by the Institute of Nuclear Medicine and Allied Sciences (INMAS) in collaboration with Hyderabad-based Dr. Reddy’s Laboratories (DRL). INMAS runs under the Defence Research and Development Organisation (DRDO).
How 2-DG Anti COVID Drug works?
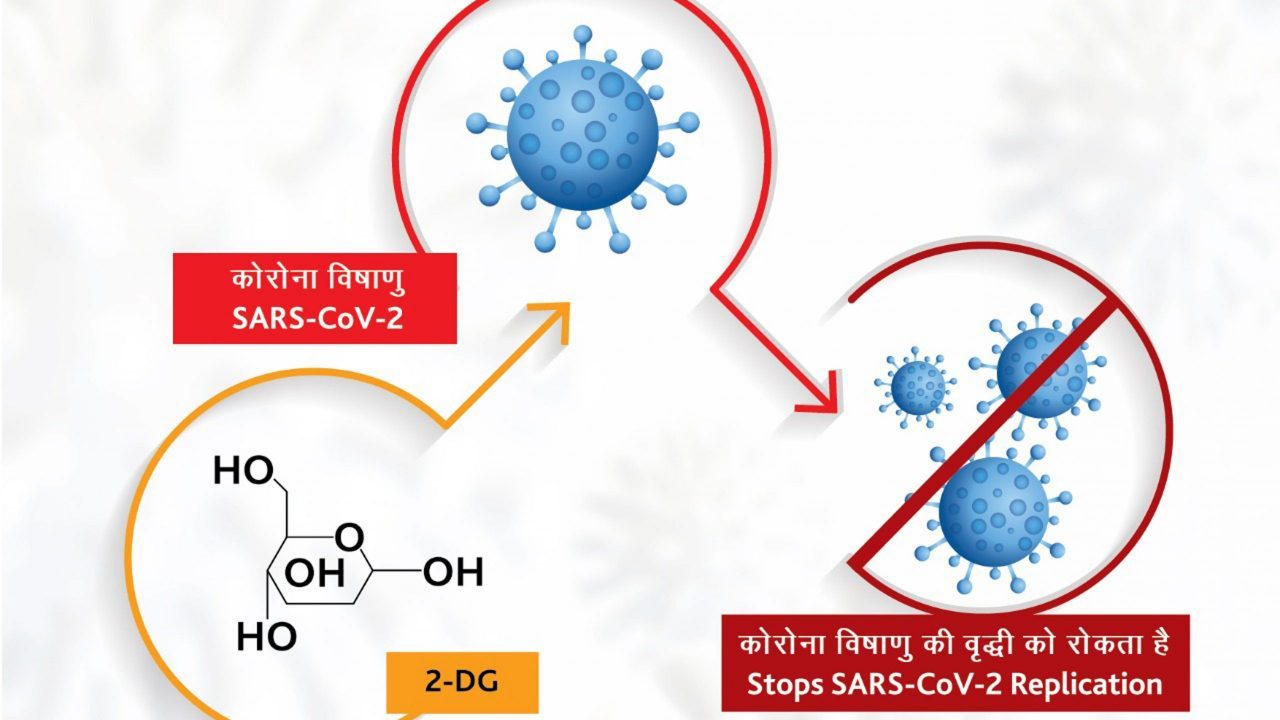
2-deoxy-D-glucose (2-DG) is a derivative of glucose, and the DRDO developed 2-DG spreads in the body like glucose. According to the government release, 2-DG prevents the growth of the virus by accumulating in the COVID infected cells and stopping viral synthesis and destroying protein-energy production. Therefore, it helps hospitalized patients of COVID-19 recover faster and reduces their dependence on supplemental oxygen.
The drug has a unique element as it provides selective accumulation in virally infected cells. The drug is in powder form, and it will be orally administered after dissolving it in water.
2-DG is generally an experimental drug as it is an anticancer and antiviral agent.
Clinical Trial and Results
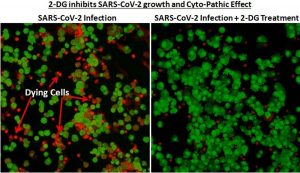
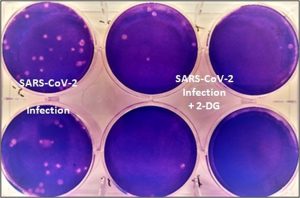
Even though there have been press releases about the anti-COVID drug, there have been no published studies about DRDO and Dr. Reddy’s Laboratories (DRL) trials yet. Due to this reason, there have been some concerns about the usage and efficacy of the drug.
Similarly, on the Clinical Trial Registry of India (CTRI) website, no corresponding entries for the mentioned Phase-II trials can be found.
Phase II
The DCGI’s Central Drugs Standard Control Organization (CDSCO) approved phase II clinical trials of 2-DG in Covid-19 patients. According to the government, DRDO and DRL conducted phase II trials on 110 patients between May and October of 2020.
The phase II results showed that patients had improved recovery. According to the release, “The patients treated with 2-DG showed faster symptomatic cure and median time of achieving normalization of specific vital signs was a significantly favorable trend.” It was done in comparison to Standard of Care (SoC).
Phase III
DCGI approved phase III clinical trials between December 2020 and March 2021 based on practical phase 2 clinical trial results. They carried the phase III trial on 220 patients in 27 COVID hospitals.
According to the Phase III findings, “In the 2-DG arm, a significantly higher proportion of patients progressed symptomatically and were free from supplemental oxygen dependency (42 percent vs. 31 percent) by Day-3 in contrast to the SoC, suggesting an early release from Oxygen therapy/dependence.”
However, as per entries in the registry-found Phase-II trial for 2-DG has only 40 participants spread over 12 sites. It is also facing criticism upon the criterion on the definition of “normalization,” along with the statistical significance of the term “significantly favorable.”
The reports also have not mentioned any possible side effects of the drug. Without the details of the clinical trial of the drug released in the public domain, experts and doctors remain doubtful about the usage and efficacy of this drug.
Also Read: Coronavirus (COVID-19) worldwide update till August